Nitrogen Cycle Process and Human Impacts
The cycle in which nitrogen is converted into different chemical forms as it move among environment, terrestrial, and marine type ecosystems. The conversion of nitrogen can be carried out through Biological fixation Process.
Requirement of Nitrogen
Nitrogen is required by all living organisms for the synthesis of organic molecules such as amino acids, nucleic acids and proteins. The Earth’s atmosphere contains almost 80% nitrogen gas.
Nitrogen in Food Chain
It cannot be used in this form by most living organisms until it has been fixed, that is reduced (combined with hydrogen), to ammonia. Green plants, the main produces of organic matter, use this supply of fixed nitrogen to make proteins that enter and pass though the food chain.
Decomposer Activity
Micro-organisms (the decomposers) break down the proteins in excretions and dead organisms, releasing ammonium ions. These two processes from part of the nitrogen cycle.
Movement of nitrogen between the earth and the atmosphere
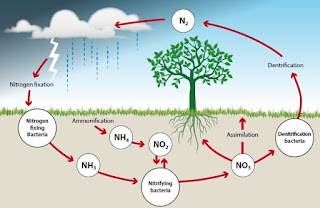
The nitrogen cycle is the movement of nitrogen between the earth and the atmosphere. It consists of a series of processes that convert nitrogen gas to organic substances and these back to nitrogen in nature. It is a continuous cycle maintained by the decomposers and other bacteria.
The nitrogen cycle can be broken down into four type of reaction and micro-organisms play roles in these entire.
Fixation of Nitrogen
Nitrogen gas is composed of two atoms of nitrogen linked by a very strong triple bond. This makes it chemically non-reactive and large amounts of energy are required break the bond. Nitrogen gas can be fixed in three ways.
Types of Nitrogen Fixation
· Atmospheric fixation: This occurs spontaneously by lightning; a small amount (5-8%) only is fixed this way. Lightening allows nitrogen and oxygen to combine to produce various oxides of nitrogen. These are carried by the rain into the soil where they can be used by plants.
· Industrial fixation: the Haber process is used to make nitrogen-containing fertilizers. This is a very energy inefficient process.
· Biological fixation: Nitrogen-fixing bacteria fix 60% of nitrogen gas in the atmosphere.
Biological fixation
The reduction of nitrogen gas to ammonia is energy intensive. It requires 16 molecules of ATP and a complex set of enzymes to break the bonds so that the nitrogen can combine with hydrogen.
Nitrogen-fixing bacteria
Only a relatively few bacteria (the nitrogen-fixing bacteria) are able to carry out this reaction. Fixed nitrogen is made available to plants y the death and lysis of free-living nitrogen-fixing bacteria or from the symbiotic association of some nitrogen-fixing bacteria with plants.
Types of bacteria for Nitrogen Fixation
Some nitrogen-fixing bacteria are free-living in the soil, fixing nitrogen independently of other organisms, e.g. Azotobacter (aerobic) and Clostridium (anaerobic).
Some nitrogen-fixing bacteria (e.g. Rhizobium) from symbiotic associations with roots of leguminous plants and appear in the form of swellings called nodules. Free-living rhizobia invade the legume through an infection thread formed in the root hair of the plant.
The infection thread is constructed by the root cells and not the bacteria and is formed only in response to infection. The infection thread grows through the root hair cells and penetrates other root cells near by.
Root nodule
often with branching of the thread. The root cells then proliferate to form a root nodule. Within a week of infection small modlules are visible to the naked eye. Each root nodule is packed with thousands of living Rhizobium bacteria.
Root-modulated non-legume is a diverse group of woody species such as alder-with e.g. Frankia. These filamentous bacteria infect the roots of plants forming actinorhizal root nodules Anabaena azollae is a cyanobacterium that infects new leaves of Azolla as they develop from the stem.
Strings of Anabaena get caught in tiny leaf hairs that grow from a dimple on the developing leaf. The dimple grows larger into a pouch-like structure that eventually closes up, locking the Anabaena inside the leaf.
Adapting to their environment
Nitrogen-fixing bacteira contains an enzyme complex called nitrogenase which catalyses the conversion of nitrogen gas to ammonia. It supplies the hydrogen as well as energy from ATP. The nitrogenase complex is sensitive to oxygen, becoming inactivated when exposed to it.
This is not a problem with the free-living anaerobic bacteria such as Clostridium. Free-living aerobic bacteria have a variety of different mechanisms for protecting the nitrogenase complex, including high rates of metabolism and physical barriers. Azotobacter overcomes this problem by having the highest rate of respiration of any organism thus maintaining a low level of oxygen in their cell.
Nitrification:
This is the oxidation of ammonium compounds to nitrites and then to nitrates by the nitrifying bacteria. During these oxidation reactions energy is released.
The nitrifying bacteria are chemoautotrophs and are able to use this source of energy to produce organic compounds from inorganic ones. (Photoautotrophs use light energy to produce organic compounds from inorganic ones.) Nitrification is a two-step process.
· Bacteria of the genus Nitrosomonas convert ammonium ions to nitrites (NO2). (Nitrite is toxic to plants and animals in high concentrations.)
· Bacteria of the genus Nitrobacter convert nitrites to nitrates (NO3). The nitrates can then be taken in by plants.
· Nitrification occurs in well drained and aerated soils at neutral pH.
Denitrification
This is the conversion of nitrates into primarily nitrogen gas but also nitrous oxide gas by the denitrifying bacteria e.g. Pseudomonas.
Denitrifying bacteria
Denitrifying bacteria transform nitrate in extremely wet soils and swampy grounds, where there is very little oxygen, i.e. the conditions are anaerobic.
The bacteria get the oxygen they need for respiration from the breakdown of nitrates. The gases that are formed escape into the atmosphere completing the nitrogen cycle. This can be a harmful process as fixed nitrogen is removed from the soil making it less fertile.
Ammonification
This is the conversation of organic forms of nitrogen (e.g. in dead organisms and their excretions) into inorganic nitrogen. A wide range of soil fungi and bacteria called the decomposers carry out the ammonification process. The decomposers consume the organic matter and the nitrogen contained in the dead organism is converted to ammonium ions. The ammonium is then converted to nitrates by the nitrifying bacteria.
Comments
Post a Comment